The self-assembly of molecular components to obtain systems and materials with structures on the nanometer scale (1 nanometer = 1 billionth of a meter) is one of the basic processes of nanotechnology. It takes advantage of the tendency of molecules to evolve to reach a state of thermodynamic equilibrium, that is, of minimum energy. However, living things function by chemical transformations that occur away from thermodynamic equilibrium and can only occur by providing external energy. Reproducing such mechanisms with artificial systems is a complex and ambitious challenge that, if met, could enable the creation of new substances, capable of responding to stimuli and interacting with the environment, which could be used to develop, for example, smart drugs and active materials.
Exploiting an ingenious combination of photochemical (i.e., light-induced) reactions and self-assembly processes, a team based at the Center for Light Activated Nanostructures (CLAN) of ISOF, led by CLAN director Alberto Credi, has succeeded in inserting an azobenzene-based filiform molecule into the cavity of a ring-shaped cyclodextrin molecule according to a high-energy geometry that is not possible at thermodynamic equilibrium. In other words, light makes it possible to create a molecular “fit” that would otherwise be inaccessible. Besides the significance for fundamental science, the results open new avenues for applications in chemical synthesis and the development of active materials and artificial systems capable of operating in biological environments with mechanisms similar to those of living organisms.
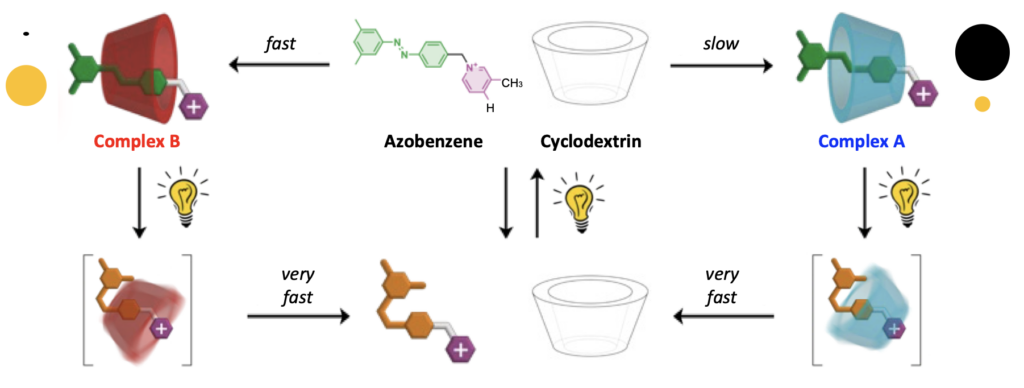
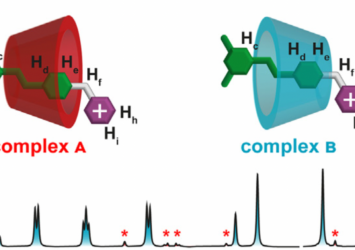
“We have shown that by administering light energy to an aqueous solution, a molecular self-assembly reaction can be prevented from reaching a thermodynamic minimum, resulting in a product distribution that does not correspond to that observed at equilibrium,” says Alberto Credi. “Such a behavior, which is at the root of many functions in living organisms, is poorly explored in artificial molecules because it is very difficult to plan and observe. The simplicity and versatility of our approach, together with the fact that visible light – i.e., sunlight – is a clean and sustainable energy source, allow us to foresee developments in various areas of technology and medicine.”
The Center for Light Activated Nanostructures (https:// centri.unibo.it/clan) is a joint research laboratory established in 2017 between CNR and the University of Bologna to perform frontier research in the areas of supramolecular chemistry, photochemistry, materials science and nanoscience. Since its foundation in 2017, the CLAN researchers published more than 80 articles in international journals, including high impact ones such as Science, Nature Nanotechnology, Chem and J. Am. Chem. Soc.
Link to full paper:
Light-driven ratcheted formation of diastereomeric host-guest systems
Neira et al., 2025, Chem 11, 102375