Better, cheaper batteries will require the synthesis of electrode materials with an optimal nano-structure. This will be crucial to enhance active surface area for high active-material utilization (thus, high capacities) and to shorten diffusion lengths for fast ion/electron transfer; however, the random dense packing and agglomeration in conventional electrodes lead to low tap density and capacity fading during cycling.
A Joint collaboration between Italy, Spain and Sweden allowed to fabricate multilayer hierarchical electrodes, where the layered structure allow for better performance.
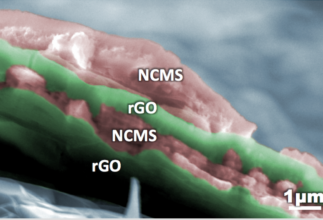
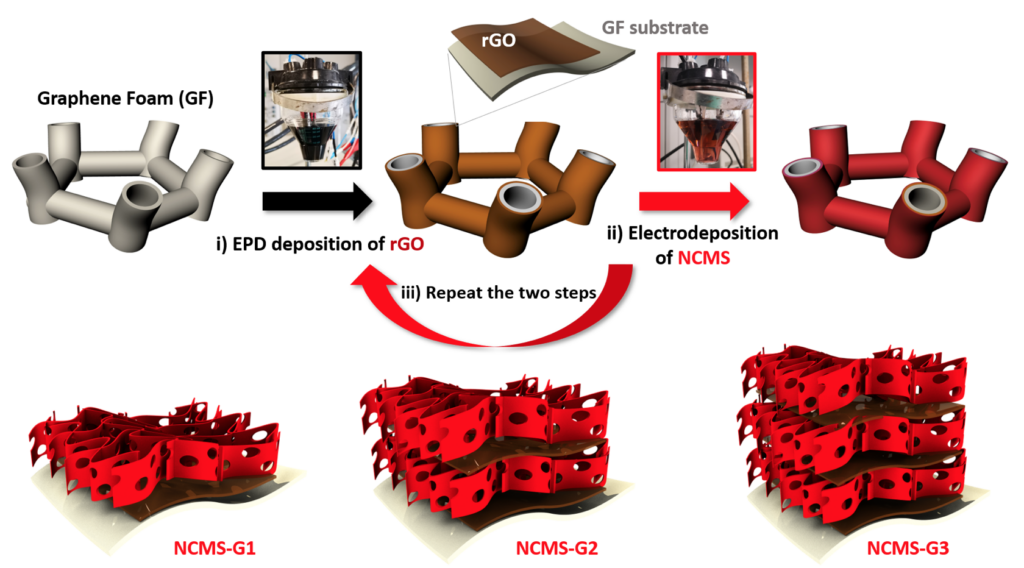
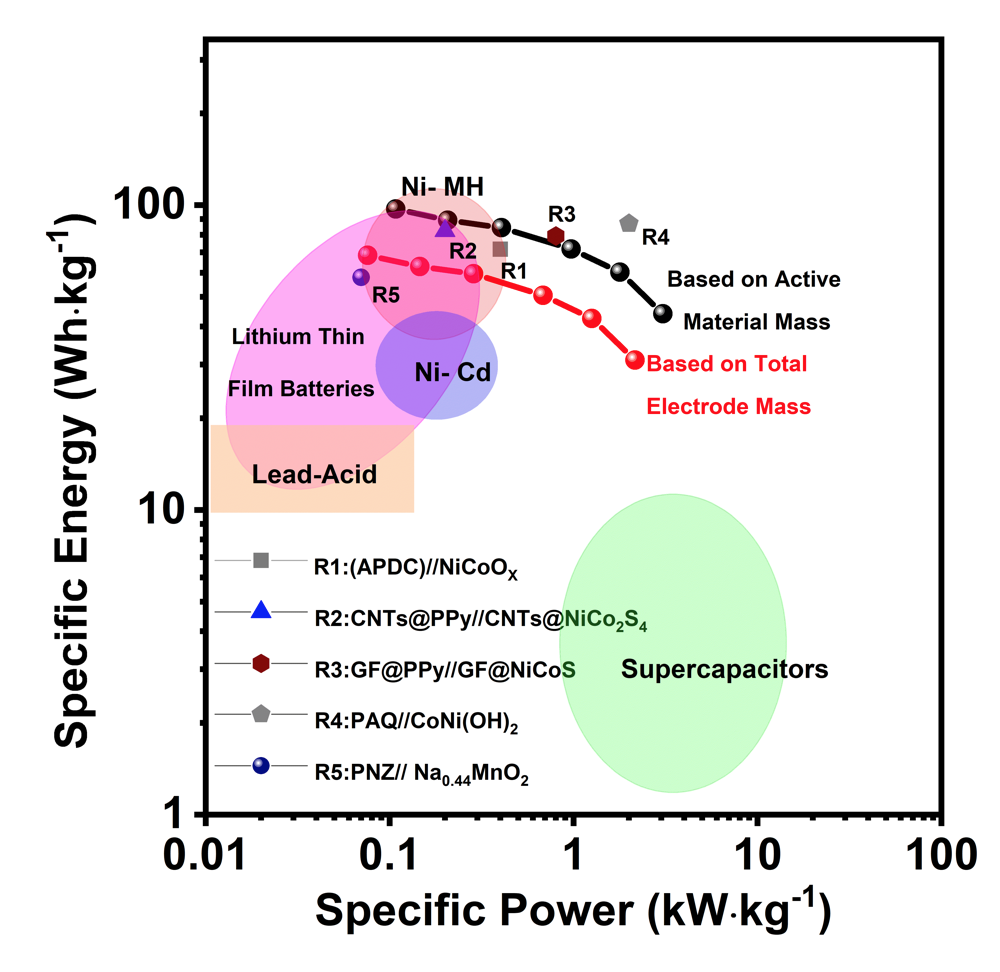
The electrodes were made of reduced graphene oxide (rGO) and mixed transition metal sulfides (NiCoMnSx), which could be deposited directly on conductive electrodes applying a small electric bias, yielding an ideal porous network and a continuous path for transport of ions and electrons. A fully rechargeable alkaline battery (RAB) was assembled with such electrodes, providing energy density of 97.2 Wh/kg and maximum power density of 3.1 kW/kg, and outstanding cycling stability (retention 72% after 7000 charge/discharge cycles at 10 A/g). The gravimetric energy density was 7 times higher than that of typical commercial supercapacitors, higher than that of Ni/Cd or lead–acid Batteries and similar to Ni–MH Batteries. The approach can be used to assemble multilayer composite structures on arbitrary electrode shapes.
Link to full article:
Jaime S. Sanchez, Zhenyuan Xia, Nagaraj Patil, Rebecca Grieco, Jinhua Sun, Uta Klement, Ren Qiu, Meganne Christian, Fabiola Liscio, Vittorio Morandi, Rebeca Marcilla, and Vincenzo Palermo*